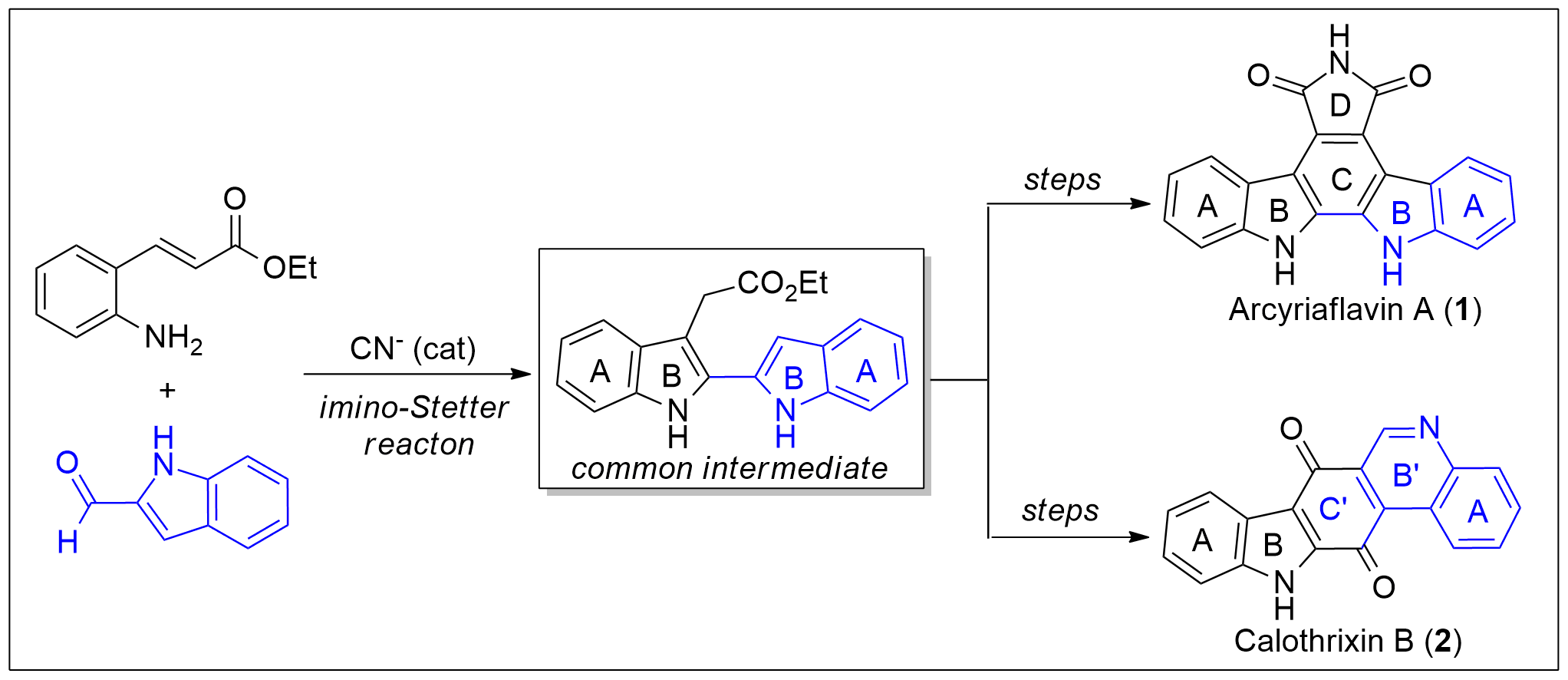
Total Syntheses of Arcyriaflavin A and Calothrixin B Using 2,2′-Bisindole-3-acetic Acid Derivative as a Common Intermediate
A new protocol for the synthesis of 2,2′-bisindole-3-acetic acid derivatives from aldimines derived from 2-aminocinnamic acid derivatives and indole-2-carboxaldehyde was developed via a cyanide-catalyzed imino-Stetter reaction. With this protocol, the divergent total syntheses of arcyriaflavin A, a representative indolocarbazole natural product, and calothrixin B, a representative indolo[3,2-j]phenanthridine natural product, were completed using a 2,2′-bisindole-3-acetic acid derivative as the common intermediate.