Ionic effects on the proton transfer mechanism in aqueous solutions
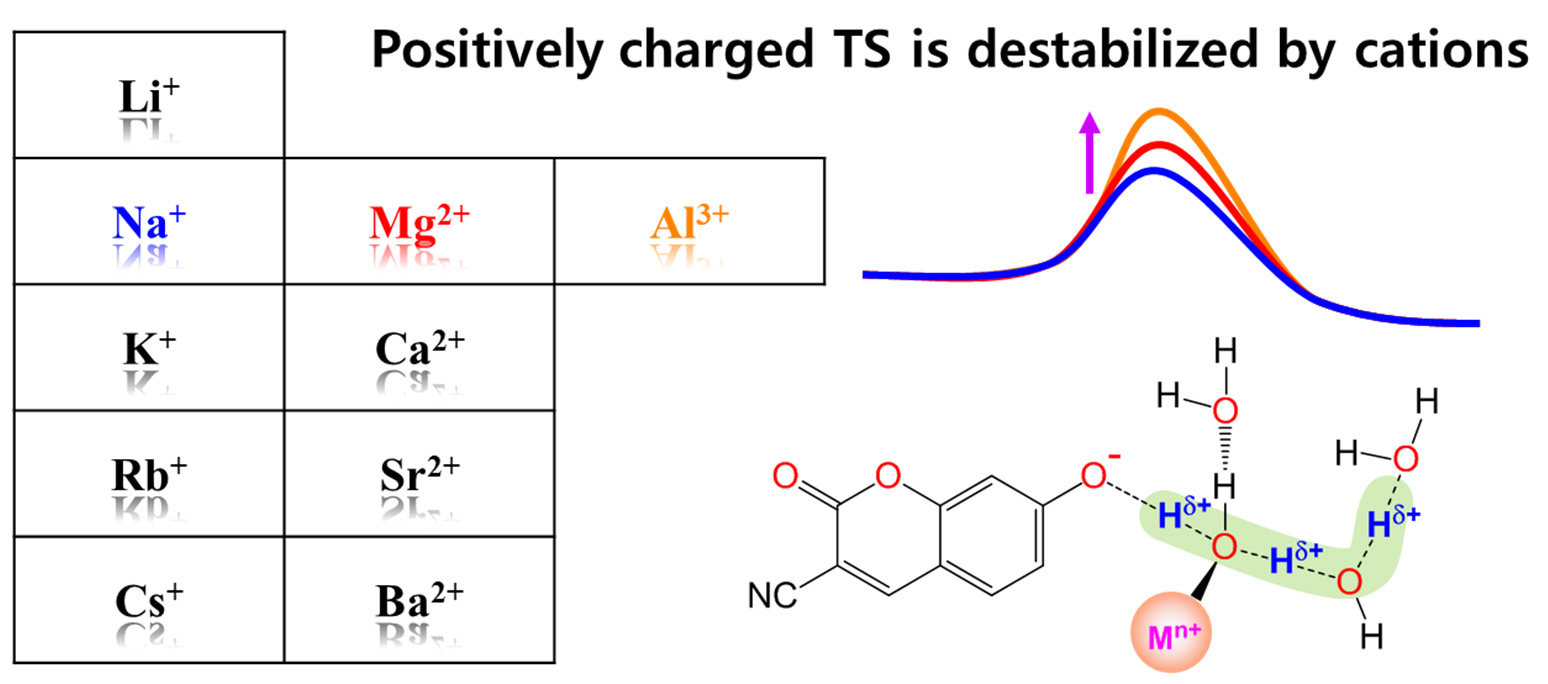
Proton dissociation (PD) reactions of weak acids and proton transfer (PT) processes in aqueous solution are strongly influenced by ions. However, a detailed molecular picture that describes how ions affect the rates for PD and PT processes is still missing. Here, we utilize time-resolved fluorescence spectroscopy combined with quantum chemical calculations to investigate the excited-state proton transfer (ESPT) reaction of a photoacid in aqueous metal chloride solutions. The activation energy (Ea) for the ESPT of the photoacid increases with increasing charge density of cations (rcat). The local H-bond structure of the photoacid in the ionic hydration shell is strongly related to both the Ea and the rcat. Most importantly, the proton’s positive charge in the transition state, which is delocalized through the H-bonded water channel, is more destabilized with an increase in the rcat, leading to a higher Ea. Our experimental and computational results allow us to elucidate the underlying mechanism for the ionic effect on PD and subsequent PT process on the molecular level.
http://pubs.rsc.org/en/content/articlelanding/2017/cp/c7cp04392a#!divAbstract