Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M=Ir and Pt) nanoframes toward efficient oxygen evolution catalysis
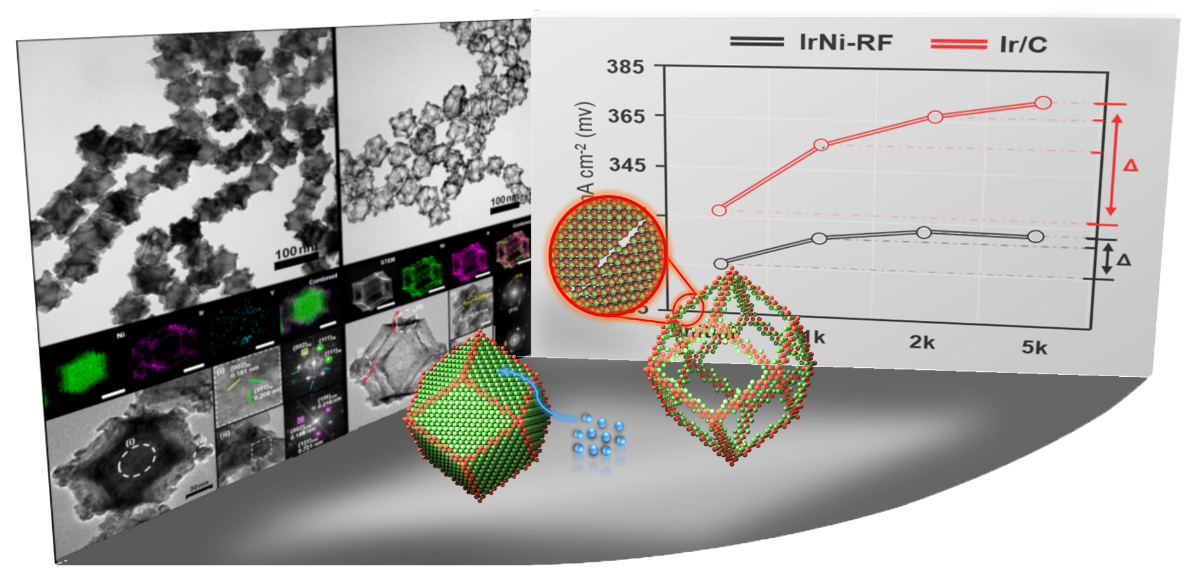
Mixed metal alloy nanoframeworks have shown a great promise as electrocatalysts in water electrolyzers and fuel cells. Although a limited number of mixed metal alloy nanoframeworks have been synthesized through phase segregation of alloy phases and removal of a component, there remains a strong need for a straightforward and facile synthesis route to this important nanostructure. A wide avenue for nanoframework structures can be opened with a fail-proof method for edge-coating shape-controlled template nanoparticles. Herein, we demonstrate that lanthanide metal chlorides can selectively passivate facets of a Ni nanotemplate, leaving the edges for the growth of a secondary metal (M= Ir, Pt). The edge-deposited metal can be further in situ mixed with the underlying Ni phase to afford rhombic dodecahedral nanoframes of binary alloy phases, namely, IrNi (IrNi-RF) and PtNi (PtNi-RF). IrNi-RF showed excellent electrocatalytic activity for the oxygen evolution reaction (OER) in an acidic electrolyte, requiring and overpotential of only 313.6 mV at 10 mA cm-2. Furthermore, even after 5000 potential cycles in the OER, IrNi-RF underwent little performance loss with an overpotential of 329.3 mV at 10 mA cm-2, demonstrating excellent catalytic stability. The presence of highly active grain boundaries, agglomeration-free frame structures, as well as the presence of IrNi/IrOx interface might be responsible for the excellent electrocatalytic activity and stability.
http://www.sciencedirect.com/science/article/pii/S2211285517306377